The supply chain for hemp ingredients like CBD is crowded, with the whole spectrum of quality available. At the top are legitimate, transparent sources exerting extreme care, control and expertise. At the bottom, a glut of traders and speculators who are blind to product provenance and production.
Supplement & Distributor SOP’s and Procedures
If you market or sell your own label of supplement or food product that is manufactured by a third party, such as a contract manufacturer or copacker, then you are responsible for its manufacture and labeling—even if you never physically touch the product.
Finding success in the RTD beverage aisle

While learning from success is seemingly more enjoyable, there are lessons to be learned in failure as well. Learn the top tips for success and avoiding failure in the RTD beverage aisle.
Blake Ebersole | Sep 17, 2020
First published in Natural Products Insider
Insider Takes
- The RTD beverage aisle in closeout stores provides valuable intel regarding product launch failures.
- World circumstances have led to great opportunities for enjoyable, affordable functional beverages.
- Brands should focus on creating a unique value proposition versus struggling to find a new ingredient.
Everyone likes to emulate and learn from the success stories in product development—the ones that made it big and struck it rich. But learning from failure is important, too. Because for every success story in ready-to-drink (RTD) beverages, a dozen struggled to turn a profit. In the internet age, products developed with great fanfare all too often disappear into oblivion within a couple years. And whatever happened to (insert the name of that drink)? Fortunately, there’s a place to see what’s ready to fall off the edge of the world.
In almost every decent-sized town and city, a closeout grocery market offers expired and overstock goods that couldn’t be sold in the primary markets. The RTD beverage aisle in closeout stores is prominent, due to its large size and great selection of drinks that no one else wanted when they were fresh. I call it the aisle of failure. This place is fascinating, because each bottle tells its own story—with its dusty caps and sedimented bottoms, its past-expiration dates, and prices marked down to a dollar or less. Their stories are about the superfruit that never really was, about the outdated formulas that ignored consumer trends and basic concepts of functional drinks. Their stories tell a lot about consumers, who are both less intelligent and smarter than is often realized.
On these shelves is a university of education for a product developer in how to keep products away from their final oblivion…
Help with life’s real issues
Today’s peri-COVID world is tiring and stressful, and that’s before turning on the TV or scrolling through one’s social media feed. Fewer people than ever have the disposable income to take a vacation or buy their next house. So, $3 for a temporary indulgence has become the Calgon bubble bath of the 2020s. The current demand has never been greater for more energy and less stress, filling in nutritional gaps, or just something satisfying that seems healthy. Today more than ever, for better or worse, enjoyment often comes in a bottle or can.
Minimize sugar and “junk”
Sure, sugar tastes good. (Really good). And it secretly wants to kill us softly with its song. And consumers, now accustomed to the taste of noncaloric sweeteners, are increasingly willing to sacrifice a little bit of that authentic sugary flavor for what they perceive as a real benefit. (And did I mention, health is top of mind now?)
Then there’s “junk,” which like beauty, is in the eye of the beholder. It’s always interesting to see each brand’s unique take on what junk means to them. But looking at a population of products in a category, repeated definitions of junk emerge, which must mean something—at least in terms of anticipated demand. The junk ingredients have funny or chemical-sounding names, and don’t state the ingredient’s natural source or purpose. At the same time, the “not junk” ingredients are typically from plants, and are often called “organic,” whatever that means in reality. Whether one agrees with the meaning of junk ingredients, it’s fair to say that in general, natural, plant-based is better. (Although isn’t sugar from a plant too? But I digress.)
Meaningfully different, yet familiar
The drink market is crowded and challenging. Sure, a brand could launch another me-too flavored water with antioxidants, but turning a profit on knockoffs requires brute strength, patient investors and a lot of luck. Meeting high-demand and up-trending needs in a new and meaningful way is the holy grail, and hitting on this combination requires a mix of familiar yet different. And still, what’s different needs to be meaningful to the consumer, and provide a clear benefit that competitors have difficulty offering.
Natural ingredients with a simple, science-based story
Imagine for a moment a new superfruit called the bubbleberry—claimed to have magical antioxidant powers. Plenty of superfruits have come and gone whose long-term value didn’t live up to their hype. Some critical questions on “hero” ingredients like the bubbleberry should be answered before their promise is burst. Namely, why should consumers care about the bubbleberry? What are its benefits, and will it make the consumer feel good about their purchase? Does the science support its claims, much less its safety for all types of people? And especially, where on Earth is this magical berry grown and processed, how is it grown and processed, and by whom?
The past few decades have brought a number of safe, clinically proven ingredients to choose from, and suppliers who have dedicated their entire business to developing them. There’s no need to find the next bubbleberry when options already exist with validated science, benefits and safety.
Avoiding the shelf of product oblivion means learning from the textbook of product failures, and understanding the rapidly changing needs of the current consumer. Let’s not forget the lessons history had to teach us—especially at the end of a product’s lifespan.

When Modern Science Collides with Ayurveda
What do traditional Ayurveda and modern science have in common — other than repeated testing of hypotheses, and communication of evidence? Answer: They both warrant

The Bad Supplements List: Failed and Prohibited Products and Brands
The Bad Supplements List: Failed and Prohibited Products and Brands. The Bad Supplements List documents and reviews clear and reliable evidence, using publicly available information on testing and enforcement activities on U.S. dietary supplement brands and products.

What’s next.. vitamin gobstoppers?
I remember the time I was called to an all-hands product development call regarding an amazing new technology. The crack team of young marketing whizzes

Smart Extracts for Smarter Supplements
The brain bone is connected to the hip bone — and just about everything else in our body. So when we think about antioxidant-rich botanicals

Dietary Supplements Prohibited by Amazon.com
The following list of supplements are banned for sale by Amazon as of April 18, 2023. This list did not provide business owner names or

New Research and Opportunities in Weight Management
Untold tens of thousands of published scientific studies reveal ways to maintain a healthy body weight. Most weight management studies published in the past 20
Product Strategy Consultants for Dietary Supplements
Product strategy for health products like supplements requires extensive knowledge of consumer trends, marketing, consumer behavior, food science, regulatory requirements and technical and scientific affairs.
NaturPro Scientific combines expertise in a wide array of product types and disciplines, offering clients a way to maximize chances for consumer product success in the market.
Product Strategy – Cores of Discipline
A product may be determined as safe and effective, and also legal and kosher — but still not be positioned for success. For example, it may be undifferentiated in a crowded market, or not provide a meaningful benefit that is valued by the consumer. A great product may find itself swimming in infested waters filled with competitors.

Our in-depth analysis and advisory process helps to guide our clients in the right direction.
Some main considerations for any supplement product design consultants includes the following:
- Target Health Category(s) and subcategories, including potential niche markets
- Market Opportunity Analysis
- Market Size & Market Leaders
- Key Competitor Analysis
- Pricing Sensitivity
- Consumer Preference Analysis, including consumner surveys and focus groups
- Key Product Benefit
- Regulatory Review
- Customer Demographics
- Differentiation, Positioning and Competitive Analysis
- Innovation Strategy
- SWOT Analaysis
- Marketing and Distribution Channel Analysis
Healthy Food and Supplement Beverage Formulators
Creating a successful healthy food or beverage is a lot more than selecting a list of ingredients that mix well together.

NaturPro has a broad base of knowledge in product development and production of dietary supplements, healthy foods and healthy beverages, spanning from raw material to finished functional food or beverage.
We guide our clients in the right direction, by helping to manage all or parts of the process for healthy food and beverage formulation and development — from seed to shelf — for dietary supplement and health food products.
Functional Food and Drink Development
Our client list includes folks of all shapes and sizes, from startup to large corporation.
No two clients or products are the same, but there are some common approaches found in our Product Development Toolbox:
Product Development Toolbox: Top 5 Product Development Tools:
Product development requires a ‘toolbox’ of analysis including the following
- Market Analysis, Competitive Analysis and Positioning
- Regulatory Status / Safety Assessment
- Claims Development and Substantiation
- Product Costing and Financials
- Ingredient Readiness, Supplier Qualification

Contact Us
- Email: [email protected]
- Website: www.npscientific.com
- Twitter: @NaturalBlake
- Instagram: @NaturPro
Pilot and Benchtop Prototype Capsules & Powders for Dietary Supplements
Creating a successful pilot and bench top prototype capsule is a lot more than creating a list of ingredients that mix well together.
NaturPro has a broad base of knowledge in capsule development and prototype formulation as well as healthy foods and natural products, spanning from raw material to finished consumer product.
We guide our clients in the right direction, by helping to manage all or parts of the process for natural product formulation and development — from seed to shelf — for dietary supplement and health food products.
Product Prototypes and Samples
Our client list includes folks of all shapes and sizes, from startup to large corporation. Many of them need small runs of new or custom products for stability and human survey trials.
No two clients or projects are the same, but there are some common approaches found in our Development Toolbox:
Imagine the Possibilities…

Product Development Toolbox: Top 10 Developer’s Tools:
Product development for dietary supplement capsules requires a ‘toolbox’ of analysis including the following
- Market Analysis, Competitive Analysis and Positioning

- Regulatory Status / Safety Assessment — GRAS | ODI | NDI




- Intellectual Property Development
- Manufacturing Feasibility
- Contract Manufacturer Qualification and Negotiation
Contact Us
- Email: [email protected]
- Website: www.npscientific.com
- Twitter: @NaturalBlake
- Instagram: @NaturPro
CBD products fail another test—with an important caveat for retailers
“These are mostly no-name brands,” said Blake Ebersole, founder and president of NaturPro Scientific, a consultancy helping companies with product development, quality compliance and manufacturing of supplements. “The products were actually purchased from convenience stores and ‘CBD shops’ as opposed to health-food stores.”
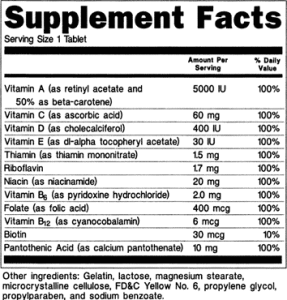
Dietary Supplement Facts and Label Review
There’s a lot of detail required for dietary supplement labels. Between supplement facts, structure-function health claims, content claims, and required formatting, it’s easy to overlook some of the FDA requirements for labeling.
Dietary Supplement Facts and Label Review
As part of our Label Review services, NaturPro helps clients develop, review and suggest improvements to dietary supplement labels, to ensure compliance with FDA regulatory requirements
Our clients enjoy the following benefits:
- Reliability: 100% accuracy and FDA compliance
- Experience: 15+ years of experience reviewing supplement labels
- Science-driven: Our reviews are based on the most current, reliable information, techniques and evidence
- Perspective: We have experience on the business side of the industry, so we know what the law is, whether it’s followed, and what is likely to happen if you don’t.
Updated Pricing:
Label Review (Basic): $350-550 — review only label — for “red flags” and suggested improvements
Label Review (Complete): $550-850 – review of label for red flags and suggested improvements, and matching finished product specifications
Label and Marketing Review: $800-1500 per label – web page / sales sheet
(Volume discounts may apply for similarly labeled products — Contact for Pricing
FDA Dietary Supplement Labeling Guidelines
See our Dietary Supplement Label Review Checklist.
The following outlines some of the most frequently asked questions (FAQ) for dietary supplement labels:
- How are dietary supplements defined?Dietary supplements are defined, in part, as products (other than tobacco) intended to supplement the diet that bear or contain one or more of the following dietary ingredients:
- A vitamin;
- A mineral;
- An herb or other botanical;
- An amino acid;
- A dietary substance for use by man to supplement the diet by increasing the total dietary intake; or
- A concentrate, metabolite, constituent, extract, or a combination of any ingredient mentioned above.Further, dietary supplements are products intended for ingestion, are not represented for use as a conventional food or as a sole item of a meal or the diet, and are labeled as dietary supplements.
- What label statements are required on the containers and packages of dietary supplements?Five statements are required: 1) the statement of identity (name of the dietary supplement), 2) the net quantity of contents statement (amount of the dietary supplement), 3) the nutrition labeling, 4) the ingredient list, and 5) the name and place of business of the manufacturer, packer, or distributor.
- Where do I place the required label statements?You must place all required label statements either on the front label panel (the principal display panel) or on the information panel (usually the label panel immediately to the right of the principal display panel, as seen by the consumer when facing the product), unless otherwise specified by regulation (i.e., exemptions).
- What label statements must I place on the principal display panel?You must place the statement of identity and the net quantity of contents statement on the principal display panel. Where packages bear alternate principal display panels, you must place this information on each alternate principal display panel.
- How do I locate the principal display panel?The principal display panel of the label is the portion of the package that is most likely to be seen by the consumer at the time of display for retail purchase. Many containers are designed with two or more different surfaces that are suitable for use as the principal display panel. These are alternate principal display panels.
- What label statements must I place on the information panel?You must place the “Supplement Facts” panel, the ingredient list, and the name and place of business of the manufacturer, packer, or distributor on the information panel if such information does not appear on the principal display panel, except that if space is insufficient, you may use the special provisions on the “Supplement Facts” panel in 21 CFR 101.36(i)(2)(iii) and (i)(5). See questions 46 and 56 in Chapter IV for more details.
- Where is the information panel?The information panel is located immediately to the right of the principal display panel as the product is displayed to the consumer. If this panel is not usable, due to package design and construction (e.g. folded flaps), the panel immediately contiguous and to the right of this part may be used for the information panel. The information panel may be any adjacent panel when the top of a container is the principal display panel.
- What name and address must I list on the label of my product?You must list the street address if it is not listed in a current city directory or telephone book, the city or town, the state, and zip code. You may list the address of the principal place of business in lieu of the actual address.
- May I place intervening material on the information panel?No. You may not place intervening material, which is defined as label information that is not required (e.g., UPC bar code), between label information that is required on the information panel.
- What type size, prominence and conspicuousness am I required to use on the principal display panel and the information panel?You are required to use a print or type size that is prominent, conspicuous and easy to read. The letters must be at least one-sixteenth (1/16) inch in height based on the lower case letter “o,” and not be more than three times as high as they are wide, unless you petition for an exemption in accordance with 21 CFR 101.2(f). The lettering must contrast sufficiently (it does not need to be black and white) with the background so as to be easy to read. See Chapter IV for the type size requirements for the nutrition label.
- Do I need to specify the country of origin if my product, or the ingredients in my product, is not from the United States?Yes. Unless excepted by law, the Tariff Act requires that every article of foreign origin (or its container) imported into the United States conspicuously indicate the English name of the country of origin of the article.
- What is the nutrition label for a dietary supplement called?The nutrition label for a dietary supplement is called a “Supplement Facts” panel.
- You must list dietary ingredients without RDIs or DRVs in the “Supplement Facts” panel for dietary supplements. You are not permitted to list these ingredients in the “Nutrition Facts” panel for foods.
- You may list the source of a dietary ingredient in the “Supplement Facts” panel for dietary supplements. You cannot list the source of a dietary ingredient in the “Nutrition Facts” panel for foods.
- You are not required to list the source of a dietary ingredient in the ingredient statement for dietary supplements if it is listed in the “Supplement Facts” panel.
- You must include the part of the plant from which a dietary ingredient is derived in the “Supplement Facts” panel for dietary supplements. You are not permitted to list the part of a plant in the “Nutrition Facts” panel for foods.
- You are not permitted to list “zero” amounts of nutrients in the “Supplement Facts” panel for dietary supplements. You are required to list “zero” amounts of nutrients in the “Nutrition Facts” panel for food.How does “Supplement Facts” differ from “nutrition facts?”The major differences between “Supplement Facts” panel and “Nutrition Facts” panel are as follows:
- What information must I list in the “Supplement Facts” panel?You must list the names and quantities of dietary ingredients present in your product, the “Serving Size” and the “Servings Per Container.” However, the listing of “Servings Per Container” is not required when it is the same information as in the net quantity of contents statement. For example, when the net quantity of contents statement is 100 tablets and the “Serving Size” is one tablet, the “Serving Per Container” also would be 100 tablets and would not need to be listed.
- How must I display the “Supplement Facts” panel?The “Supplement Facts” nutrition information (referred to as a panel) must be enclosed in a box by using hairlines. The title, “Supplement Facts,” must be larger than all other print in the panel and, unless impractical, must be set full width of the panel. The title and all headings must be bolded to distinguish them from other information.
- How must I present the information in the “Supplement Facts” panel?You must present all information using the following:
- A single easy-to-read type style;
- All black or one color type, printed on a white or neutral contrasting background, whenever practical;
- Upper- and lowercase letters, except that you may use all uppercase lettering on small packages (i.e., packages having a total surface area available to bear labeling of less than 12 square inches);
- At least one point leading (i.e., space between lines of text); and
- Letters that do not touch.
- What are the type size requirements for the “Supplement Facts” panel?Except as provided for small and intermediate-sized packages, you must set information other than the title, headings, and footnotes in uniform type size no smaller than 8 point. You also must use a type size larger than all other print size in the nutrition label for the title “Supplement Facts.” You may set the column headings and footnotes in type no smaller than 6 point type. See the section on “Special Labeling Provisions” for the exceptions for small and intermediate-sized packages.
For more information, visit FDA Dietary Supplement Labeling Guide
Supplement contract manufacturing recall ‘unprecedented’
Blake Ebersole, founder and president of NaturPro Scientific LLC, a consulting firm in the natural products industry, lauded FDA for its ultimate enforcement action. “Companies like this are a big drag on the industry and they’re putting out potentially unsafe products, giving responsible companies a black eye,” he said in an interview.
Dietary Supplement Recall
January 17, 2020 — Dietary Supplement Recall announced for 1,200 products from 850 supplement distributors made between January 2013 and November 2019.

ABH NATURE’S PRODUCTS, INC, ABH PHARMA, INC., and STOCKNUTRA.COM, INC. (the “COMPANIES”) is conducting a nationwide recall of ALL lots of its dietary supplement products pursuant to a Consent Decree entered by the U.S. District Court for the Eastern District of New York. This recall applies to all dietary supplement products manufactured and sold between January 2013 – November 2019 and all lots of products are included in this recall.
These products are being recalled after an FDA inspection found significant violations of current good manufacturing practice regulations. Manufacturing practices that are not in adequate control represent the possibility of risk being introduced into the manufacturing process resulting in finished supplement products with decreased identity, purity, strength and composition.
To date, there have been no reported illnesses or injuries as a result of this situation.
The COMPANIES contract manufactured dietary supplements for other firms and did not sell products directly to consumers. Link to Press Release
Contract Manufacturing Advocacy and Recall Prevention
It’s important to recognize the differences between GMP certification and GMP verification.
If you own a supplement brand, and are not 100% sure whether your supplement contract manufacturer is meeting FDA requirements, contact us.