The Bad Supplements List: Failed and Prohibited Products and Brands
Last revised: March 1, 2024
ABOUT THE BAD SUPPLEMENTS LIST:
The Bad Supplements List documents and reviews clear and reliable evidence, using publicly available information on testing and enforcement activities on non-compliant U.S. dietary supplement brands and products. The List focuses on recent reports of firms, brands and products which may still be present in the market.
Source information on this page is not confidential, and is freely available for public view online. Information may be provided by government, public or private entities with permission.
Bad Supplements is a working document, and will be continuously updated.
HOW TO VIEW:
1. Search by product or company name using Find (Ctrl-F) or the search box at the bottom of the page.
2. Scroll down through this page to the following sections
- NOW Foods Testing of Dietary Supplements
- Flagrant Violations, Court Orders and Injunctions
3. Visit the List of Prohibited Supplements on Amazon.com
DISCLOSURES:
NaturPro Scientific has no financial interest in any dietary supplement brand or product. We do not accept payment for removal from or inclusion on this List. Bad Supplements is made possible with support from responsible firms in the dietary supplement industry. Please contact us to learn how to support the Bad Supplements List.
Information posted by NaturPro Scientific LLC on the Bad Supplements List or elsewhere are based on an expert review based on reasonable interpretation of regulatory requirements and mainly public information. Private information may be posted, only with permission from the owner. We do not disclose any information which is prohibited from public view, or considered confidential or proprietary.
Although we take responsibility for reviewing information herein to reliable sources, we are not responsible for the accuracy of information provided by any third parties. Information on this page may be updated or changed without notice.
We recommend all supplement consumers to verify all brand and product information with the brand owner, and to research enforcement action on all brands and products before consuming. Recommended search terms typically include the regulatory agency (e.g. FDA, FTC) and the brand or product name.
Some sources cited may require access or subscription fees from the publisher.
NOW Foods Testing Program for Mislabeled Supplement Products
46% of Creatine Gummies Fail Label Claim (February 29, 2024)
In its latest round of testing competing supplement, supplement company NOW found widespread failings to meet label claims when testing several creatine gummies it purchased online. This exercise reached a second red flag when, following the testing program’s usual practice of simultaneous testing by a reputable third-party lab, none of the outside labs NOW vetted and approved were capable of testing gummies.
A survey of several brands of creatine was performed to understand the quality available on the marketplace, which included the brands Astro Labs, Beast Bites, Create, Con-Cret, Greabby, and Njord who failed to meet the label claim, resulting in a 46% failure rate.
Gummy brands testing below label claim were also tested for creatinine using HPLC. Several creatine gummies were found to contain significant amounts of this unwanted creatine metabolite, while also not meeting their claimed creatine strength. Creatinine is a waste product that naturally builds up in blood when muscles are exercised. Bodies produce creatinine at a constant rate, and kidneys usually eliminate almost all of it. Having very high or low creatinine levels can be a health concern; creatinine supplements are not recommended. Creatine in powder form is stable, but when mixed with water can turn into creatinine. Gummies are not an ideal form for creatine supplements because water is used to make gummies, so it can be difficult to get the correct dosage of creatine, NOW reports.
Astro Labs, Greabby, and Njord had a small amount of creatinine detected, while Beast Bites, Create, and Con-Cret had large amounts of creatinine present. Taking the creatine and creatinine data together shows that Astro Labs, Greabby, and Njord likely had a minimal amount of creatine, almost all of which converted to creatinine.
Source: https://www.nutraceuticalsworld.com/contents/view_breaking-news/2024-02-29/now-reports-widespread-failings-in-creatine-gummy-tests/?
26 of 33 Berberine Products on Amazon and Walmart.com Don’t Contain Labeled Amount of Berberine (December 20, 2023)
In the 16th round of product testing since 2017, NOW tested more than 30 Berberine supplements after surveying the online marketplace for questionable products and identified serious quality problems from “no name” brands sold on Amazon and Walmart.com.
- The products chosen were purchased from both Amazon and Walmart.com in early November 2023. These brands were picked because they are less known and sold almost entirely on these platforms. (We have chosen not to test health food store brands or practitioner brands as our experience shows them to be less of a concern.)
- The results of this round of testing showed serious levels of low potencies with every brand testing below 100% potency, except NOW. Seven brands did contain over 80% labelled potency and three brands contained 90-97% potency.
- NOW has tested some of the same brands previously, while testing other categories, and found similar problems.
- Eighteen of the 33 brands tested contained less than 40% of labelled potency. That’s more than half of all brands tested that didn’t even contain a mediocre 40% level of potency.
- Seven of the 33 tested samples had 1% or less of Berberine potency in each product. All of these seriously flawed brands were tested for the first-time in NOW’s testing programme and may be new brands.
Source: https://nutraceuticalbusinessreview.com/berberine-test-results-reveal-serious-quality-problems-from
14 of 21 Astaxanthin Products on Amazon Contain Lower than Labeled Amount (August 24, 2023)
We performed a preliminary investigation into the identity of the 14 brands failing NOW Foods astaxanthin potency testing… so here’s a quick and dirty breakdown of the cheaters.
1. About half of the brands are not associated with a physical address.
And out of the ones I could find a physical address for, only one address has a building large enough to produce supplements. According to Google Maps, most brands that had a physical address were located inside an unrelated business, or a residence like an apartment or house.
2. About half of the brands have no website.
Those without websites only have an Amazon Seller and/or Walmart seller page with no contact information.
3. Thirteen of fourteen brands don’t list the name of an owner or person responsible.
(Don’t you hate when the “About Us” page says nothing about who’s behind the brand, and the “Contact Us” page has no address or phone number? Too bad, because that’s the new normal.)
4. ZERO Amazon-only Seller pages listed a person’s name, business address or phone number.
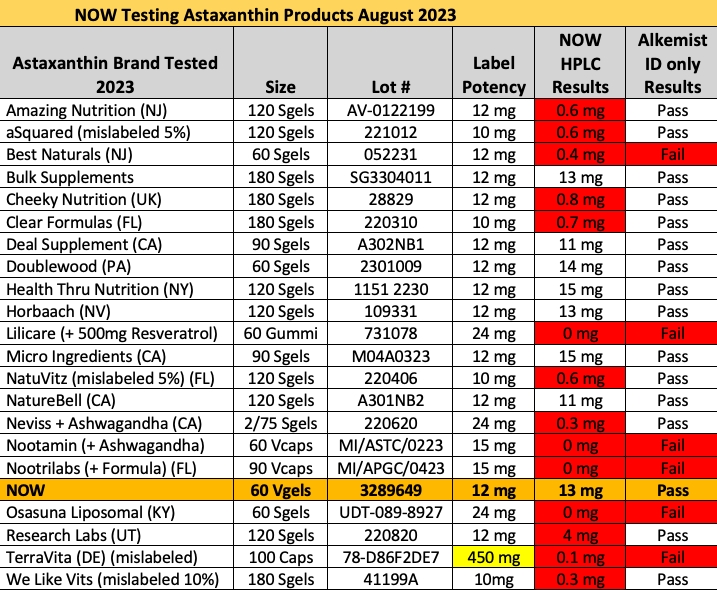
As reported in Nutraceuticals World in August 2023, 13 of 22 supplement brands tested by NOW Foods from Amazon Sellers contain less than 10% of the labeled amount of astaxanthin.
Astaxanthin is generally an expensive ingredient, and manufacturers are often interested to cut costs on testing or production when no one is looking.
AMAZON ASTAXANTHIN PRODUCT MISLABELED RESULTS, PER SERVING
Amazing Nutrition: contains 0.6 mg versus 12 mg labeled
aSquared: contains 0.6 mg versus 10 mg labeled
Best Naturals: contains 0.4 mg versus 12 mg labeled
Cheeky Nutrition: contains 0.8 mg versus 12 mg labeled
Clear Formulas: contains 0.7 mg versus 12 mg labeled
Lilicare: contains 0 mg versus 24 mg labeled
NatuVitz: contains 0.6 mg versus 10 mg labeled
Neviss: contains 0.3 mg versus 24 mg labeled
Nootamin: contains 0 mg versus 15 mg labeled
Nootrilabs: contains 0 mg versus 15 mg labeled
Osasuna: contains 0 mg versus 24 mg labeled
Research Labs: contains 4 mg versus 12 mg labeled
Terra Vita: contains 0.1 mg versus [incorrect dosage] labeled
We Like Vitamins: contains 0.3 mg versus 10 mg labeled
15 of 20 Bromelain Supplements Fail Potency (March 1, 2023)
- 15 out of 20 samples tested (75%) failed to meet label claims for potency. Only six out of 20 samples contained over 50% of labeled potency.
- Shocking that 12 out of 20 brands contained less than even 10% of label claim! That means more than half of all tested Bromelain supplement samples contained less than one-tenth of label claims — the most egregious testing results NOW has seen!
- Three samples had ambiguous labeling that only claimed weight and no activity. Generally, Bromelain powder has an activity of 2,400 GDU per gram, but these brands made no potency claims. Two of these products’ potencies were below the detection limit and were reported as < 10 GDU/g. The third brand, Cadane, only contained 34 GDU of Bromelain per capsule, which is extremely low potency.
- Three brands were labeled as products made in India. These brands tested to only contain 10, 34 and 78 GDU potencies, respectively. Two were under 10% of label claim and the third made no potency claim.
- Only four brands exceeded 100% potency with NOW as one at 121%.
- NOW intentionally did not test known “natural” or practitioner brands. The reason being these other brands typically abide by Good Manufacturing Practices (GMPs) and we expect full potencies.
- NOW has been testing products sold by lesser-known brands for six years and consistently finds most suspect products failing potency testing. We have shared this information widely, with the goal of addressing bad quality products in the marketplace. Unfortunately, testing shows the problem is getting worse.
- NOW has been testing products exclusively found on Amazon, but we will begin adding unknown products found on Walmart.com in the future. We are finding that low potency products often spend the most dollars marketing on both Amazon and Walmart.com. Two of the top four “sponsored” or paid advertised brands from a search on Amazon in March 2023 included the violating brands below.
⚠︎ = Brands that had 100%+ potencies
|
Brand Tested for Potency |
Size |
Lot # |
Label Potency |
GDU Claim |
Total GDU/Cap |
Test Results |
% Label Claim |
|
Amazing Nutrition (NJ) |
120 Vcaps |
AV-21120??? |
500mg |
2,400 |
1,200 |
24 GDU/cap |
2% |
|
Balance Greens (MI) |
180 Caps |
VM2200267/1 |
500mg |
2,400 |
1,200 |
12 GDU/cap |
1% |
|
Best Naturals (NJ) |
120 Tabs |
12251 |
500mg |
600 |
300 |
17 GDU/tab |
6% |
|
Best Vite (CA) |
120 Vcaps |
110621 |
500mg |
2,400 |
1,200 |
96 GDU/cap |
8% |
|
Cadane (India) + Formula |
60 Vcaps |
CRBO6C01/1 |
300mg |
? |
? |
34 GDU/cap |
n/a |
|
Deal Supplement (CA) |
180 Caps |
21-388 |
500mg |
300 |
150 |
126 GDU/cap |
84% |
|
Earthborn Elements (OR) |
200 Caps |
76255 |
575mg |
? |
? |
< 10 GDU/cap |
0% |
|
Fresh Nutrition (CA) |
90 Vcaps |
B21224006-1 |
208mg |
2,400 |
500 |
608 GDU/cap |
122% |
|
H&C (India) |
180 Vcaps |
NCBE102 |
250mg |
2,400 |
600 |
10 GDU/cap |
2% |
|
⚠︎ Horbaach |
120 Caps |
123369 |
850mg |
600 |
480 |
690 GDU/cap |
144% |
|
Herbal Secrets (NJ) |
120 Tabs |
AN-22101702 |
500mg |
300 |
150 |
37 GDU/cap |
25% |
|
⚠︎ NOW |
120 Vcaps |
3277931 |
500mg |
2,400 |
1,200 |
1451 GDU/cap |
121% |
|
⚠︎ Nutricost (UT) |
120 Vcaps |
22102614 |
500mg |
2,400 |
1,200 |
1202 GDU/cap |
100% |
|
NutriONN (OR) |
120 Vcaps |
B989710 |
500mg |
2,400 |
1,200 |
39 GDU/cap |
3% |
|
NUSA Pure/GMAX (NC) |
150 Vcaps |
2206038 |
750mg |
300 |
225 |
20 GDU/cap |
9% |
|
Pure Supplements (UT) |
100 Caps |
73628 |
575mg |
? |
? |
< 10 GDU/cap |
0% |
|
⚠︎ Superior Labs (UT) |
120 Vcaps |
2202025 |
500mg |
2,400 |
1,200 |
1321 GDU/cap |
110% |
|
TenMid (India) + Formula |
90 Vcaps |
TBRO9C01/1 |
350mg |
2,400 |
840 |
78 GDU/cap |
9% |
|
Vitamatic (NJ) |
180 Caps |
P2100020 |
500mg |
2,400 |
1,200 |
815 GDU/cap |
68% |
|
We Like Vitamins (TX) |
180 Vcaps |
BR8958-138-3 |
500mg |
2,400 |
1,200 |
< 10 GDU/cap |
0% |
Source: https://www.nowfoods.com/quality-safety/testing-results-bromelain-supplements-purchased-amazon
7 of 8 CoQ10 Supplements Fail Potency Testing (April 2022)
NOW continued its award-winning industry self-policing program of testing unfamiliar brands found on Amazon and, unfortunately, the cheating continues. The program, begun in 2017, tests high value products sold by unheard of brands on Amazon at both internal and external labs, and evaluates the results compared to label claims.
NOW reexamined eight brands of CoQ10 to see if those that were identified as low potency in testing done in 2020 had improved and found the same serious problems remain for seven out of eight brands tested. Additionally, as shown below, NOW found brands cheating by misrepresenting potencies through deceptive labeling tricks.
NOW purchased three samples of each product below and tested by HPLC both internally at NOW’s state-of-the-art labs and externally at the highly regarded Eurofins labs. It is apparent by looking at lot numbers and bottle types that the same manufacturer is supplying multiple brands with the same fraudulent products (see Florida brands in the chart below).
- Clear Formulas, aSquared, Foxy Doc and Healthy Way brands all mislabel their product as “400mg/6%” potency. This is deceptive when the front panel says “400mg” potency and the Amazon title says “CoQ10 400mg Max Strength”. The customer gets less than 24mg CoQ10 per capsule.
- NOW previously tested a variety of CoQ10 brands on Amazon in 2017, 2018 & 2020 with similar failing results. aSquared, Healthy Way, NasaBe’Ahava and We Like Vitamins were all under 35% potency in 2020 as well.
- Seven out of eight brands tested had less than 30% of the potency claimed
- Perhaps most alarming, three of the eight brands claimed to be in vegetarian capsules, but testing both at NOW and at Eurofins confirmed gelatin was used. The failing brands are Clear Formulas, Healthy Way and Sundhed.
Amazon Brands April 2022 Size Lot # Label Claim/Cap NOW Results Eurofins Av %
⚠︎ aSquared Nutrition, FL 100 Vcaps 30465 400 mg/6% 20 mg 23.6 mg Mislabel
⚠︎ Clear Formulas, FL 200 Vcaps 30620 400 mg/6% 23 mg 23.3 mg Mislabel
⚠︎ Foxy Doc, PA 200 Vcaps 30611A 200 mg/6% 9 mg 10.9 mg Mislabel
⚠︎ Healthy Way FL 200 Vcaps 30442 200 mg/6% 11 mg 12.4 mg Mislabel
⚠︎ NasaBe’Ahava, FL 200 Vcaps 30662 200 mg 11 mg 12.1 mg 6%
NutriONN, OR 120 Vcaps CQ210710 200 mg 199 mg 198 mg 99%
⚠︎ SUNDHED, FL 60 Vcaps 30520 400 mg 21 mg 21.8 mg 5%
⚠︎ We Like Vitamins, TX 120 Vcaps CQ60050 200 mg 54 mg 53.4 mg 27%
2022 Results found at NOW Foods website
NOW Foods Testing of Magnesium Supplements from Amazon.com (2022)
Magnesium Glycinate has become a very popular form of Magnesium and we tested 16 total samples. NOW discovered that almost all other brands tested failed to include the chelated magnesium form, as claimed on the label. Magnesium chelates, such as magnesium bisglycinate or glycinate, have excellent water solubility and lack a laxative effect. The fully reacted chelates are better absorbed and more expensive than other forms and thus, are at risk for substitution with lower quality material, such as magnesium oxide and magnesium carbonate, simply blended with glycine.
Various magnesium glycinate products, including two manufactured by NOW, were purchased on Amazon and subjected to testing at our in-house lab, as well as at Eurofins contract laboratory. First, total magnesium content was determined by analyzing the samples using Inductively Coupled Plasma – Optical Emission Spectrometry (ICP-OES). To determine the levels of water-soluble magnesium glycinate, the same ICP-OES technology was used, but instead of acid digestion, a gentle water extraction was applied to all samples. Twelve out of 16 tested products met the label claim when we tested total magnesium content. However, only NOW products met the label claim, when we looked at the soluble (chelated) form of magnesium, suggesting that other brands use non-soluble forms of magnesium in place of the more expensive chelated form. Glycine was also detected in all samples, although the soluble magnesium results clearly suggest that glycine was not (or not entirely) bound with magnesium. Unfortunately, it is known in the industry that many brands either knowingly or unknowingly simply blend glycine with magnesium oxide or carbonate and then label the product as “Magnesium Glycinate”. The difference is that the improperly labeled product is much lower cost and is not a fully reacted or bonded chelate. The results are summarized in the table below.
Additionally, it seems some brands mislabel intentionally in order to get higher label potency claims. Deal Supplement brand claims 750mg of “Magnesium Glycinate” per capsule, while legal labeling should list the elemental dose of Magnesium and not the total weight of a RDI ingredient. Other brands that mislabel in this same way include: Innate Vitality, Naturebell, Terranics and ZYY Nutrition. Most brands label Magnesium Glycinate properly, such as below:
Magnesium (as Bisglycinate)…..125mg or Magnesium (from Glycinate)…..125mg
Or Magnesium 200mg (from 2,000mg Magnesium Bisglycinate) as NOW does for full disclosure of total and elemental mineral weights.
We intentionally did not test most brands that claimed to use Albion Minerals**, which are known to be high quality and specialists in fully reacted Magnesium Glycinate. Magnesium Bisglycinate Chelate powder from Albion Minerals/Balchem contains 10% elemental magnesium. This is why it takes 2,000mg of Magnesium Bisglycinate powder to yield 200mg of elemental Magnesium in NOW brand. Some brands such as Toniiq claim that their Magnesium Glycinate is 20% elemental, but this can only be achieved by using Magnesium and Glycine that are not fully reacted/bonded together. NaturaLife appears to be labeled properly with 18% elemental Magnesium potency with Albion as the source, but this also is a blend – “buffered” – in order to reach the higher potency claim. So NaturaLife is accurate because they include “Magnesium Oxide” in the side panel of ingredients, but also deceptive since the front panel only lists Magnesium Glycinate. Horbaach brand seems to accurately label their side panel as: Magnesium 240mg (from 1,330mg Magnesium Glycinate Chelate – Magnesium Bisglycinate Chelate, Magnesium Oxide). But this is also deceptive since the front panel claims 1,330mg per serving and the side panel states that Magnesium is only 240mg. This form of Magnesium Glycinate contains a blend with Magnesium Oxide and claims to be made “with chelated minerals”, but not 100% chelate.
Test Results December 1, 2022
**https://balchem.com/human-nutrition-health/hnh-products/albion-minerals/
⚠︎ = potency claims not met or poor results
| Brand Tested Mag Glycinate Supplements Dec 2022 | Size | Lot # | /Front Panel Claim | Sice Claim/Cap/Tab | NOW Total Magnesium Results | Eurofins Total Magnesium Results | NOW Soluble Magnesium Results | Eurofins Soluble Magnesium Results |
|---|---|---|---|---|---|---|---|---|
| NOW Mag Bisglycinate Powder | 8 oz | 3256627 | n/a | 250 mg/serv | 273 | 265 | 271 | 260 |
| NOW Mag Glycinate 100mg | 180 Tabs | 3258879 | n/a | 100 mg/tablet | 111 | 110 | 111 | 101 |
| ⚠︎ Deal Supplement CA | 200 Caps | P2207015 | 750 mg | 750 mg/cap | ⚠︎ 131 | ⚠︎ 126 | ⚠︎ 75 | ⚠︎ 63 |
| ⚠︎ Doublewood PA | 180 Vcaps | 220257 | 400 mg | 60 mg/cap | 69 | 71 | ⚠︎ 39 | ⚠︎ 28 |
| ⚠︎ Dr. Martin’s Nutrition NV | 180 Vcaps | 405458 | 425 mg | 212.5 mg/cap | 298 | 285 | ⚠︎ 85 | ⚠︎ 64 |
| ⚠︎ Horbaach NV | 250 Caps | 120520 | 1300 mg | 120 mg/cap | 130 | 128 | ⚠︎ 62 | ⚠︎ 49 |
| ⚠︎ Innate Vitality CA | 120 Vcaps | P220702 | 500 mg | 500 mg/cap | ⚠︎ 65 | ⚠︎ 62 | ⚠︎ 52 | ⚠︎ 38 |
| ⚠︎ Nature’s Branch FL | 200 Tabs | 2220527 | 400 mg | 28 mg/tab | 30 | 30 | ⚠︎ 18 | ⚠︎ 16 |
| ⚠︎ NaturaLife Labs CA | 120 Vcaps | 220808 | 450 mg | 150 mg/cap | 162 | 160 | ⚠︎ 64 | ⚠︎ 58 |
| ⚠︎ Natures Craft NY | 90 Vcaps | 2022-14474 | n/a | 133.3 mg/cap | 172 | 179 | ⚠︎ 14 | ⚠︎ 12 |
| ⚠︎ Naturebell CA | 180 Caps | P2206027 | 500 mg | 500 mg/cap | ⚠︎ 67 | ⚠︎ 73 | ⚠︎ 52 | ⚠︎ 41 |
| ⚠︎ Purely Holistic UT | 270 Tabs | 2022-14260 | 400 mg | 200 mg/tab | 196 | 197 | ⚠︎ 47 | ⚠︎ 44 |
| ⚠︎ Terranics CA | 120 Vcaps | P2201007 | n/a | 500 mg/cap | ⚠︎ 47 | ⚠︎ 50 | ⚠︎ 47 | ⚠︎ 40 |
| ⚠︎ Toniiq IL | 240 Caps | 22F0021 | n/a | 150 mg/cap | 167 | 178 | ⚠︎ 68 | ⚠︎ 49 |
| ⚠︎ Whollium MA | 90 Tabs | 2207-015A | n/a | 150 mg/tab | 152 | 150 | ⚠︎ 127 | ⚠︎ 111 |
| ⚠︎ ZYY Nutrition CA | 180 Caps | P2203002 | 1000 mg | 500 mg/cap | ⚠︎ 59 | ⚠︎ 65 | ⚠︎ 49 | ⚠︎ 39 |
NOW Foods Testing of Turmeric and Curcumin Supplements (2021)
Continuing the work to disclose alarming quality failings and misleading, inaccurate labels among unfamiliar brands of products purchased on Amazon, NOW has turned their attention to the curcumin/turmeric category, finding similarly poor results.
Having previously tested multiple categories of suspect supplements purchased on Amazon that revealed low potency and poor quality control, NOW recently tested turmeric extracts for potency, heavy metals, labeling accuracy, and potential addition of synthetic curcumin.
NOW assayed 23 unknown brand product samples plus two NOW products, all purchased from Amazon in June 2021. Initially it appeared that the unknown brands tested much better than expected. Only one product clearly failed potency testing and four others tested very low, but without any specific label claim. This initial look was not the end of the story.
Virtually all of the products were labeled such as “Turmeric Curcumin 1650mg” on the front panel, yet the side panel would list 1,500mg as Turmeric Root, 300mg Ginger Root, 150mg Turmeric Extract and 15mg BioPerine PER THREE CAPSULES. That equals to 655mg per capsule and less than 10% of the product is Turmeric 95% Standardized Extract. “This can be perceived as deceptive since many customers do not know the difference between Turmeric, Turmeric Extract, Curcumin Extract, and Standardized 95% Extract,” said Dan Richard, NOW’s Vice President of Global Sales and Marketing.
NOW also tested heavy metals (Arsenic, Cadmium, Lead, and Mercury) to compare each product. The average total heavy metals, by product, were 525% higher than NOW’s two sample average, and only one product out of 23 had less heavy metals than NOW. Two products, B’Leaf Nature and Eagle brands, were more than 20 times higher than NOW and above California’s Prop 65 limits for Lead. Two others, Farm Haven and BioEmblem brands, were both above 100ppb in Cadmium, a particularly toxic heavy metal.
Synthetic adulteration was also an issue in these tests. Low quality curcumin (turmeric extract) is known to be spiked with synthetic curcumin in order to meet potency testing. The American Botanical Council highlighted this problem, and quality brands have made sure to avoid it. Synthetic curcumin is derived from petrochemicals, rather than natural Turmeric, at a much lower cost. NOW sent all samples to the University of Georgia’s Center for Applied Isotope Studies for independent radiocarbon testing. This lab found that four out of 23 unfamiliar brands were spiked, with “fossil fuel derived organic carbon.” These four brands are: Vitpro, Me First Living, Eagle, and Primal Harvest.
NOW also suspected that some of these products may be mislabeled as Vegetarian Capsules. Of the 23 samples below, two tested to be in animal gelatin capsules and were not in Vegetarian Capsules. These are Bioganix and Nutriflair brands with below lot numbers.
All of these products were tested for potency both at the company’s highly respected internal labs and at Eurofins Labs. The assay method is RP-HPLC with UV detection and potencies are determined based on total curcuminoids per capsule.
In total, 12 out of 23 outside products tested failed either for potency, containing synthetic curcuminoids, heavy metals or used gelatin caps instead of the claimed veggie caps. Somewhat surprisingly, 11 out of 23 did pass all tests, though with slightly misleading labeling.
NOW is the only brand to list both total and net weights of 95% extract on labels. NOW’s # 4638 is labeled showing 665mg Turmeric Extract providing 630mg or 95% Curcumin as the primary active potency.
“While we appreciate Amazon’s initial efforts to address these ongoing, egregious problems with sellers on their platform, there is clearly still a long way to go,” Richard said. “The kind of results we found are not what consumers expect when they purchase dietary supplements from sellers they trust.”
⚠︎ = potency claims not met or poor results
| Curcumin/Turmeric 95% Tests by NOW July 2021 | Size | Lot # | Expir. Date | Label Claim 95% Extract | NOW Results # 1 Test | Eurofins Results | Av % Result |
|---|---|---|---|---|---|---|---|
| NOW Turmeric Curcumin #4638 | 60 Vcaps | 3187526 | 12/2024 | 665 mg | Pass | Pass | 107% |
| NOW Turmeric Curcumin Softgels #4936 | 60 Gels | 3206552 | 4/2024 | 475 mg | Pass | Pass | 102% |
| Ace Nutrition NJ Turmeric Curcumin | 90 Vcaps | 2020-07476 | 06/2023 | 200 mg | Pass | Pass | 102% |
| BioEmblem/ANZA NY Turmeric Curcumi | 90 Vcaps | 1B2201 | 02/2022 | 50 mg | Pass | Pass | 145% |
| ⚠︎ Bioganix FL Turmeric Curcumin | 120 Vcaps | 62053 | 01/2023 | No claim | 13 mg | 10 mg | |
| Bio Schwartz NY Turmeric Curcumin | 90 Vcaps | T20C163 | 12/2022 | 50 mg | Pass | Pass | 141% |
| B’Leaf FL Turmeric Curcumin | 180 Vcaps | 20L0251 | 10/2022 | 50 mg | Pass | Pass | 140% |
| ⚠︎ Doctor Recommended Turmeric Curcumin | 180 Vcaps | TC5151 | None | 208 mg | Fail | Fail | 3% |
| Eagle Supplements PA Turmeric Complex | 60 Vcaps | 2001007 | 01/2022 | 50 mg | Pass | Pass | 98% |
| Farm Haven CA Turmeric Curcumin | 120 Vcaps | 12988 | 07/2022 | 50 mg | Pass | Pass | 140% |
| ⚠︎ FineVine NJ Turmeric Curcumin | 180 Vcaps | None | 01/2024 | Not clear | 21 mg | 21 mg | |
| Me First Living AZ Turmeric Curcumin | 60 Vcaps | 007068 | 11/2022 | 500 mg | OK | OK | 96% |
| MedChoice CA Turmeric Bioperine Garlic | 120 Vcaps | 21C042 | 04/2023 | 50 mg | Pass | Pass | 117% |
| Nature’s Base TX Turmeric & Ginger | 60 Vcaps | 20L044 | 12/2022 | 50 mg | Pass | Pass | 99% |
| Natures Nutrition UT Turmeric Curcumin | 120 Vcaps | 11747-120 | 09/2023 | 50 mg | Pass | Pass | 150% |
| Natures Wellness FL Turmeric Curcumin | 120 Vcaps | A1B006 | 02/2024 | 50 mg | Pass | Pass | 139% |
| Naturewise CA Turmeric Curcumin | 180 Vcaps | T20H075 | 02/2024 | 167 mg | Pass | Pass | 105% |
| New Age Turmeric Curcumin (no address) | 90 Vcaps | 11904 | 02/2024 | 50 mg | Pass | Pass | 162% |
| ⚠︎ NutriFlair WA Turmeric | 180 Vcaps | TC59034 | 02/2024 | Not clear | 8 mg | 8 mg | |
| Primal Harvest NY Turmeric Complex | 60 Vcaps | 012221107 | 03/2023 | 200 mg | Pass | Pass | 119% |
| ⚠︎ Pure by Nature GA Turmeric Curcumin | 180 Vcaps | T58657 | 12/2023 | Not clear | 18 mg | 16 mg | |
| Vimerson Health IL Turmeric Curcumin | 60 Vcaps | 2021-1119 | 03/2023 | 50 mg | Pass | Pass | 142% |
| Vita Breeze FL Turmeric Curcumin | 180 Vcaps | TC59145 | 03/2024 | Not clear | 91 mg | 97 mg | |
| VitPro NJ Turmeric Curcumin | 90 Vcaps | STTCBLK321 | 03/2023 | 150 mg | Pass | Pass | 113% |
| Viva Naturals NY Organic Turmeric | 90 Tabs | 0540B9 | 09/2021 | 50 mg | Pass | Pass | 137% |
NOW Foods Testing of Phosphatidylserine Purchased on Amazon.com (2021)
Following NOW’s report earlier this year of CoQ10, Acetyl-l-Carnitine and SAMe supplements with alarming quality and labeling problems sold on Amazon, NOW has identified similar serious failings in the Phosphatidyl Serine (PS) category.
NOW purchased and tested a group of PS products on Amazon, choosing this particular supplement for several key reasons. According to SPINS data, NOW is the leading seller of Phosphatidyl Serine in natural food stores, and they know the product well. NOW staff noticed that their PS sales were declining on Amazon, and investigated. They found that the top selling brands on Amazon were offering prices that averaged 70% below NOW’s already discounted prices. Knowing that the prices looked “too-good-to-be-true”, it made sense to test and verify potencies. Because PS is an expensive supplement, the incentive is high for marketing brands to put extra funds into Amazon-sponsored fees. NOW had previously tested examples in this and other categories and found these semi-private label brands sold exclusively on Amazon to display faulty labeling and be of poor quality. When most quality brands sell 100mg PS as the standard potency, it’s questionable to see high potencies such as 400mg or 500mg.
NOW’s initial analysis of simply reading label information found multiple problems. Phosphatidyl Serine is a complicated supplement, which makes it easier to have confusion. Many of the brands included in this report list a high potency, such as 500mg, on the front label (and Amazon’s keyword) panel, yet the side panel often states that amount per 2 or 3 capsules. Others were more deceptive by adding things such as “Phosphatidyl Serine Complex” in order to show a high potency value, even when the actual potency of PS would only be 20% of that number. Here are some examples:
- Absonutrix is deceptively labeled as “Phosphatidylserine Complex 500mg”
- Naturebell is deceptively labeled as “Phosphatidylserine 20% 400mg per 2 Cap serving”. Havasu brand also labels this way “Phosphatidyl Serine 20% 100mg”
- longlifenutri is mislabeled as “Phosphatidylserine 500mg (per 2 caps) (standardized to 20%)”
- NUSA brand label is unclear how much Phosphatidyl Serine is in each capsule
- Both Earth Natural brands mislabel listing Phosphatidyl Serine as 500mg on the front, but only 50mg per Cap on the side fine print
- Correct labeling should be “Phosphatidyl Serine 100mg (from 500mg Sunflower/Soy Lecithin Complex)” if the raw ingredient is 20% PS from Complex, or simply “Phosphatidyl Serine….100mg”
A total of 43 samples were purchased on Amazon, generally three bottles of each brand, and tested to verify results. Three of these were NOW brand and results on these were 100% – 108% of label claims. Of the remaining non-NOW brands, two samples were discarded when results revealed 2.5 times the label potency claim so it was suspected these products were spiked with added Serine in order to fool testing. Two samples passed potency (not included in this report), while 36 samples failed. Of the 36 failures listed below, 17 of these products contained less than 10% of the labeled value.
“That’s a shockingly poor failure rate, but not really a surprise based upon our previous experience testing supplements sold on Amazon,” said Dan Richard, Vice President of Global Sales and Marketing. “Amazon has to find a way to raise the bar on products they sell, especially today with people buying more supplements from Amazon in order to stay healthy.”
The results are reported in the chart below, which NOW tested by HPLC both by their in-house labs and independently at Eurofins Labs (www.eurofinsus.com).
Phosphytidal Serine Assay Results May – July 2020 from Amazon Purchases April 3, 2020 and May 27, 2020.
⚠︎ = potency claims not met or poor results
| Brand Phosphytidal Serine on Amazon | Count | Lot # | Expir. Date | Label Claim per cap | Results per cap | Lab Test | % Label Claim |
|---|---|---|---|---|---|---|---|
| NOW Foods | 60 Vcaps | 3138361 | 12/2021 | 100 mg | 104.4 mg | NOW | 104% |
| NOW Foods | 120 Vcaps | 3146319 | 02/2022 | 100 mg | 100.8 mg | NOW | 100% |
| NOW Foods | 120 Vcaps | 3146319 | 02/2022 | 100 mg | 108 mg | Eurofins | 108% |
| ⚠︎ Absonutrix (mislabeled) | 120 Vcaps | AS101918-1 | 10/2021 | 520 mg | 2 mg | NOW | <2% |
| ⚠︎ Absonutrix (mislabeled) | 120 Vcaps | AS101918-1 | 10/2021 | 520 mg | 5.2 mg | NOW | <2% |
| ⚠︎ Absonutrix (mislabeled) | 120 Vcaps | AS101918-1 | 10/2021 | 520 mg | 5.8 mg | Eurofins | <2% |
| ⚠︎ BoostCeuticals (+ Formula) | 100 Caps | 5121926 | 03/2024 | 167 mg | 12.7 mg | NOW | 8% |
| ⚠︎ BoostCeuticals (+ Formula) | 100 Caps | 27012004 | 03/2024 | 167 mg | 57 mg | Eurofins | 34% |
| ⚠︎ Doublewood Supplements | 120 Caps | 1912697 | 01/2023 | 150 mg | 67.4 mg | NOW | 45% |
| ⚠︎ Doublewood Supplements | 120 Caps | 1912697 | 01/2023 | 150 mg | 48.1 mg | NOW | 32% |
| ⚠︎ Doublewood Supplements | 120 Caps | 1912697 | 01/2023 | 150 mg | 24.3 mg | Eurofins | 16% |
| ⚠︎ Dr. Maxwell (+ Bacopa ext,) | 120 Vcaps | P856510 | 01/2023 | 150 mg | zero | NOW | <2% |
| ⚠︎ Dr. Maxwell (+ Bacopa ext,) | 120 Vcaps | P856510 | 01/2023 | 150 mg | zero | NOW | <2% |
| ⚠︎ Dr. Maxwell (+ Bacopa ext,) | 120 Vcaps | P856510 | 01/2023 | 150 mg | 1.7 mg | Eurofins | <2% |
| ⚠︎ GLS Nutrition Formula/Earth Natural | 200 Caps | 11919 | 08/2021 | 250 mg/50 mg | 31.5 mg | NOW | 13% |
| ⚠︎ Havasu Nutrition + Ginkgo 120 mg | 60 Caps | 1019010 | 10/2021 | 100 mg/20% | 58.8 mg | NOW | 59% |
| ⚠︎ Havasu Nutrition + Ginkgo 120 mg | 60 Caps | 1019010 | 10/2021 | 100 mg/20% | 17.9 mg | Eurofins | 18% |
| ⚠︎ Intelligent Labs | 90 Vcaps | PS55398 | 05/2022 | 100 mg | 17.8 mg | NOW | 18% |
| ⚠︎ Intelligent Labs | 90 Vcaps | PS55398 | 05/2022 | 100 mg | 19.5 mg | NOW | 20% |
| ⚠︎ Intelligent Labs | 90 Vcaps | PS55398 | 05/2022 | 100 mg | 29 mg | Eurofins | 29% |
| ⚠︎ longlifenutri | 180 Vcaps | 6175 | 11/2022 | 250 mg/20% | 68 mg | NOW | 27% |
| ⚠︎ longlifenutri | 180 Vcaps | 6156 | 03/2023 | 250 mg/20% | 31.5 mg | NOW | 13% |
| ⚠︎ longlifenutri | 180 Vcaps | 6156 | 03/2023 | 250 mg/20% | 37.3 mg | Eurofins | 15% |
| ⚠︎ Mental Refreshment (+ Formula) | 200 Caps | 16012005 | 02/2022 | 167 mg | 136.8 mg | NOW | 82% |
| ⚠︎ Mental Refreshment (+ Formula) | 100 Caps | 22012005 | 03/2022 | 167 mg | 47.3 mg | Eurofins | 28% |
| ⚠︎ Mono Herb (plus Flax Powder) | 90 Vcaps | 03/2022 | 200 mg | 13.2 mg | NOW | 7% | |
| ⚠︎ Mono Herb (plus Flax Powder) | 90 Vcaps | 03/2022 | 200 mg | 1.4 mg | Eurofins | <2% | |
| ⚠︎ Naturebell (mislabeled) | 180 Caps | A911NB2 | 11/2021 | 200 mg/20% | 34 mg | NOW | 17% |
| ⚠︎ Naturebell (mislabeled) | 180 Caps | A002MB7 | 02/2022 | 200 mg/20% | 28 mg | NOW | 14% |
| ⚠︎ Naturebell (mislabeled) | 180 Caps | A002MB7 | 02/2022 | 200 mg/20% | 5.6 mg | Eurofins | 3% |
| ⚠︎ Naturetition Formula/Earth Natural | 200 Caps | 416-128 | 04/2022 | 250 mg/50 mg | 11.9 mg | NOW | 5% |
| ⚠︎ Naturetition Formula/Earth Natural | 200 Caps | 416-128 | 04/2022 | 250 mg/50 mg | 3.8 mg | NOW | <2% |
| ⚠︎ Naturetition Formula/Earth Natural | 200 Caps | 416-128 | 04/2022 | 250 mg/50 mg | 2.3 mg | Eurofins | <2% |
| ⚠︎ NUSA (+ Formula) | 200 Vcaps | 2004023 | 04/2023 | 250 mg ? | 31.7 mg | NOW | 13% |
| ⚠︎ NUSA (+ Formula) | 200 Vcaps | 2004023 | 04/2023 | 250 mg ? | 2.4 mg | Eurofins | <2% |
| ⚠︎ Superior Health (+ Formula) | 100 Caps | 12041911 | 08/2023 | 250 mg | 7 mg | NOW | 3% |
| ⚠︎ Superior Health (+ Formula) | 100 Caps | 12041911 | 08/2023 | 250 mg | 15.9 mg | NOW | 6% |
| ⚠︎ Superior Health (+ Formula) | 100 Caps | 12041911 | 08/2023 | 250 mg | 3.9 mg | Eurofins | <2% |
| ⚠︎ We Like Vitamins(+ Formula) | 100 Caps | 11101916 | 11/2023 | 167 mg | 138.3 mg | NOW | 83% |
For more information on the NOW Foods testing program, please visit: https://www.nowfoods.com/healthy-living/articles/now-uncovers-quality-issues-supplement-brands-sold-amazon
Warning Letters, Court Orders and Injunctions
Defyned Brands / 5 Star Nutrition LLC (January 12, 2024)
Defyned Brands, an Austin, Texas, company also known as 5 Star Nutrition LLC, pleaded guilty today to a three-count information charging it with distributing misbranded dietary supplements.
Pursuant to the plea agreement, the company admitted that from September 2018 to July 2020, it delivered into interstate commerce misbranded dietary supplements, which are considered a type of food under the federal Food, Drug and Cosmetic Act (FDCA). The company specifically admitted that shipments of products known as Epivar, Alpha Shredded and Laxobolic were misbranded. According to the plea agreement, the products contained ingredients mislabeled as dietary ingredients or not listed on the product label.
The products at issue were marketed as workout supplements and sold at 5 Star Nutrition retail locations. As part of the plea, the company agreed to forfeit $4.5 million and comply with the terms of a compliance program and certain compliance reporting requirements. Magistrate Judge Susan Hightower of the U.S. District Court for the Western District of Texas presided over the plea.
Source: https://www.justice.gov/opa/pr/texas-company-pleads-guilty-distributing-misbranded-dietary-supplements-and-agrees-45
Amazon.com (December 20, 2023)
FDA WARNING LETTER EXCERPT
Andy Jassy, CEO
Amazon.com, Inc.
2021 7th Ave
Seattle, WA 98121-2601
RE: 662503
Dear Mr. Jassy:
This letter concerns your firm’s distribution of products that violate the Federal Food, Drug, and Cosmetic Act (the “FD&C Act”). The United States Food and Drug Administration (FDA) purchased on your website, www.amazon.com, products that are labeled as energy enhancing supplements or food, but laboratory analyses confirmed that they contained undeclared and potentially harmful active pharmaceutical ingredients…
FDA purchased “MANNERS Energy Boost,” “Round 2,” “WeFun,” “Genergy,” “Big Guys Male Energy Supplement,” “Mens Maximum Energy Supplement,” and “X Max Triple Shot Energy Honey” through your website, www.amazon.com. All of these products were introduced or delivered for introduction into interstate commerce by Amazon via your Fulfillment by Amazon service.1 FDA confirmed through laboratory analyses that the “MANNERS Energy Boost,” “Round 2,” “Genergy,” and “X Max Triple Shot Energy Honey” products, purchased on www.amazon.com, contained the active pharmaceutical ingredient (API) tadalafil; and the “WeFun,”2 “Big Guys Male Energy Supplement,” and “Mens Maximum Energy Supplement” products, also purchased on www.amazon.com, contained the API sildenafil. These ingredients are not declared on the products’ labeling.3 Sildenafil and tadalafil are phosphodiesterase type-5 (PDE-5) inhibitors and the active ingredients in the FDA-approved prescription drugs Viagra and Cialis, respectively, used to treat erectile dysfunction (ED). These undeclared ingredients may interact with nitrates found in some prescription drugs, such as nitroglycerin, and may lower blood pressure to dangerous levels.
Source: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/amazoncom-inc-662503-12202023
Hua Da Trading (December 20, 2023)
FDA WARNING LETTER EXCERPT
Daniel Zeng
Hua Da Trading, Inc.
PO Box 40517
Brooklyn, NY 11204
RE: 664359
Dear Daniel Zeng:
This letter is to advise you that the United States Food and Drug Administration (FDA) reviewed your website at the Internet address, https://www.eshoponlineusa.com, from July to December 2023, where you take orders for “WeFun.”1 In addition, FDA has obtained a sample and labeling of your “WeFun” product. As described below, this product is an unapproved new drug sold in violation of sections 505(a) and 301(d) of the Federal Food, Drug, and Cosmetic Act (FD&C Act), 21 U.S.C. 355(a) and 331(d). Furthermore, your “WeFun” is a misbranded drug under section 502 of the FD&C Act, 21 U.S.C. 352, and sold in violation of section 301(a) of the FD&C Act, 21 U.S.C. 331(a).
FDA confirmed through laboratory analysis that a sample of your “WeFun” product contains the undeclared pharmaceutical ingredient sildenafil. Sildenafil is a phosphodiesterase type-5 (PDE-5) inhibitor and the active ingredient in the FDA-approved prescription drug Viagra used to treat erectile dysfunction (ED). This undeclared ingredient may interact with nitrates found in some prescription drugs, such as nitroglycerin, and may lower blood pressure to dangerous levels. Men with diabetes, high blood pressure, high cholesterol, or heart disease, often take nitrates.
Source: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/hua-da-trading-inc-664359-12202023
Artri King / Botanical Be Warning Letter (December 20, 2023)
Edgar H. Felix
Botanical Be
360 Jardin Bello
El Paso, TX 79932-2357
RE: 671066
Dear Mr. Felix:
This letter is to advise you that the United States Food and Drug Administration (FDA) reviewed your website at the Internet address https://botanical-be.com/ in July 2023, where you take orders for “Kuka Flex Forte,” “Reumo Flex,” and “Artri King Reforzado con Ortiga y Omega 3” products. In addition, FDA has obtained samples and labeling of your “Kuka Flex Forte,” “Reumo Flex,” and “Artri King Reforzado con Ortiga y Omega 3” products. As described below, these products are unapproved new drugs sold in violation of sections 505(a) and 301(d) of the Federal Food, Drug, and Cosmetic Act (FD&C Act), 21 U.S.C. 355(a) and 331(d). Furthermore, the products are misbranded drugs under section 502 of the FD&C Act, 21 U.S.C. 352 and sold in violation of section 301(a) of the FD&C Act, 21 U.S.C. 331(a).
FDA confirmed through laboratory analysis that a sample of your “Kuka Flex Forte,” “Reumo Flex,” and “Artri King Reforzado con Ortiga y Omega 3” contains undeclared diclofenac. Diclofenac is a non-steroidal anti-inflammatory drug (commonly referred to as NSAIDs). NSAIDs may cause increased risk of cardiovascular events, such as heart attack and stroke, as well as serious gastrointestinal damage, including bleeding, ulceration, and fatal perforation of the stomach and intestines. This hidden drug ingredient may also interact with other medications and significantly increase the risk of adverse events, particularly when consumers use multiple NSAID-containing products.
Total Body Nutrition LLC, TBN Labs LLC, and Loud Muscle Science, LLC, Mohammed Islam (December 18, 2023)
A civil complaint filed on October 18, 2023 at the request of the U.S. Food and Drug Administration (FDA), alleged that Total Body Nutrition LLC, TBN Labs LLC, and Loud Muscle Science, LLC (collectively, “TBN companies”), and the companies’ owner, Mohammed Islam, violated the FDCA at the companies’ facility in Hauppauge, Long Island, and their previous facility in Edgewood, New York, by manufacturing and distributing adulterated and misbranded dietary supplements. The complaint alleges that Islam and the TBN companies violated the FDCA by manufacturing dietary supplements without establishing product specifications for the finished batches and without testing or examining the finished batches to verify that they met product specifications, and by using dietary ingredients in their dietary supplements without first testing or examining the ingredients to verify their identity. The complaint also alleged that FDA inspected the TBN companies’ current and previous facilities four times, in 2017, 2018, 2021, and 2023, and found violations of the FDCA at each inspection. According to the complaint, FDA also issued Islam and the TBN companies warning letters in 2016, 2017, and 2019.
Islam and the TBN companies agreed to settle the suit and be bound by a consent decree of permanent injunction. The negotiated consent decree entered by the court enjoins Islam and the TBN companies from violating the FDCA, and requires, among other things, that Islam and the TBN companies comply with the dietary supplement current good manufacturing practice regulations and the dietary supplement labeling provisions of the FDCA and its implementing regulations. Further, Islam and the TBN companies must destroy all of their adulterated dietary supplements.
Balance of Nature and Evig LLC, and Ryan Petersen (November 16, 2023)
In a complaint filed on Oct. 11 in the U.S. District Court for the District of Utah at the request of the U.S. Food and Drug Administration, the United States alleged that Evig LLC and the company’s CEO, David Lex Howard, violated the federal Food, Drug and Cosmetic Act (FDCA) by distributing adulterated and misbranded dietary supplements. In a separate complaint filed the same day, the United States alleged that Premium Productions LLC and the company manager’s, Ryan Petersen, violated the FDCA by manufacturing adulterated dietary supplements. According to the complaints, the dietary supplements involved are marketed throughout the United States under the brand name Balance of Nature.
The U.S. District Court for the District of Utah, Central Division has entered two consent decrees of permanent injunction against Evig LLC, of St. George Utah, and the company’s CEO, Douglas Lex Howard, as well as Premium Production LLC, of St. George, Utah, and its Manager, Ryan Petersen.
The complaint against Evig LLC and Howard alleges that the defendants claimed their dietary supplements can cure, treat and prevent a variety of diseases and health conditions, including cancer, heart disease, diabetes and coronavirus. According to the complaint, the supplements were neither approved by FDA nor exempt from approval, making them unapproved new drugs and misbranded under the terms of the FDCA. The complaint further alleges that FDA inspections showed the defendants had no system in place to handle customer complaints, despite receiving reports asserting that their products may have caused allergic reactions from ingredients not identified on the label.
The complaint against Premium Productions LLC and Petersen alleges that the defendants’ operation did not follow required current good manufacturing practices and failed to develop good operating procedures and adequate quality controls, making their products adulterated under the FDCA.
FDA sent both companies warning letters in August 2019 explaining that their conduct did not comply with the FDCA. According to the government’s complaints, the defendants failed to take appropriate steps to come into compliance after receiving those letters.
Source: https://www.justice.gov/opa/pr/court-enjoins-two-utah-companies-distributing-and-manufacturing-adulterated-and-misbranded
Pharmasol Corporation and Marc L. Badia (December 14, 2023)
The U.S. District Court for the District of Massachusetts has entered a consent decree of permanent injunction ordering Pharmasol Corporation, a Massachusetts-based company, and President Marc L. Badia to stop distributing drugs until the company complies with the Federal Food, Drug, and Cosmetic (FD&C) Act and other requirements listed in the consent decree. According to the complaint, which was filed along with the consent decree by the U.S. Department of Justice, Pharmasol and Badia unlawfully distributed adulterated drugs, meaning they do not comply with manufacturing quality requirements within the U.S. marketplace.
Pharmasol manufactures and distributes over-the-counter drugs, as well as human and animal prescription drugs such as topical corticosteroids and inhalant anesthetics. Pharmasol is under contract with multiple pharmaceutical companies.
According to the complaint, the defendants violated federal law under the FD&C Act by introducing drugs into interstate commerce that fail to comply with current good manufacturing practice requirements; therefore, these drugs are adulterated.
The most recent inspection of the company’s facilities in 2022 found the majority of the inspectional observations repeated those found in past FDA inspections and detailed in a 2019 warning letter. Violations mentioned in the complaint include failure to:
- fully investigate errors and ensure that the responsibilities and procedures applicable to the quality control unit are in writing and fully followed, including reporting drug defects to customers;
- follow written procedures that describe the handling of written and oral complaints regarding a drug product; and,
- adequately clean and maintain equipment.
Source: https://www.fda.gov/news-events/press-announcements/federal-court-enters-consent-decree-against-pharmasol-distributing-adulterated-drugs
GCHNC LLC dba Hemp XR/Gate City Hemp dba Hemp XR/Allaziya Enterprises, LLC dba Hemp XR (September 28, 2023)
FDA WARNING LETTER EXCERPT
3741 Battleground Ave., Ste C
Greensboro, NC 27410
RE: # 656057
Dear Alaa Odeh Mahmoud Hamed and Abdulraouf B. Allamandani:
This letter is to advise you that the U.S. Food and Drug Administration (FDA) reviewed your website at the Internet address https://hemp-xr.com/ in September 2023 and has determined that you take orders there for various products, which you represent as containing Delta-8 tetrahydrocannabinol (THC) or cannabidiol (CBD).
FDA has determined that your Far Out Candy 500MG Delta 8 Cookies, Not Ya Son’s Weed Bakedies Delta 8 THC 600MG Crispy Bites, Hemp XR Delta 8 Stoner Candy Gummies (including Crawlers, Fruit Smashers, Magic Marbles, and Stoney Headz Sour), Lava Rocks 250 MG Delta 8, Delta 8 Rainbow Rope, Pharma Delta 8 Gummies, Hemp XR Delta 8 Honey 500 MG, and Hemp XR CBD Honey 500 MG products are adulterated under section 402(a)(2)(C)(i) of the Federal Food, Drug, and Cosmetic Act (the FD&C Act), 21 U.S.C. 342(a)(2)(C)(i), because they bear or contain an unsafe food additive. It is a prohibited act to introduce adulterated food into interstate commerce under section 301(a) of the FD&C Act, 21 U.S.C. 331(a). Furthermore, it is a prohibited act to introduce your Hemp XR CBD Honey 500 MG product into interstate commerce under section 301(ll) of the FD&C Act, 21 U.S.C. 331(ll).
Source: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/gchnc-llc-dba-hemp-xrgate-city-hemp-dba-hemp-xrallaziya-enterprises-llc-dba-hemp-xr-656057-09282023
InnoMark Inc. (September 1, 2023)
Russell M. Moody CEO/Owner
InnoMark, Inc.
4041 S. River Rd.
Saint George, UT 84790
Ref: CMS Case # 657518
Dear Mr. Moody:
The U.S. Food and Drug Administration (FDA) conducted an inspection of your facility, located at 4041 S. River Rd., Saint George, Utah, from March 8, 2023 through March 15, 2023. Based on inspectional findings and review of the labels collected during the inspection, we identified significant violations of the Federal Food, Drug, and Cosmetic Act (the Act) and applicable regulations. You can find the Act and FDA regulations through links on FDA’s home page at www.fda.gov.
We received written correspondence from you dated March 30, 2023 to the Form FDA 483, Inspectional Observations, issued to you at the close of the inspection. We address your response below, in relation to the applicable violation.
Adulterated Dietary Supplements
During the inspection, our investigators found significant violations of Title 21, Code of Federal Regulations (CFR), Part 111 (21 CFR Part 111), Current Good Manufacturing Practice (cGMP) in Manufacturing, Packaging, Labeling, or Holding Operations for Dietary Supplements. These violations cause the (b)(4), and (b)(4) sticks dietary supplements manufactured at your facility to be adulterated within the meaning of section 402(g)(1) of the Act [21 U.S.C. § 342(g)(1)] in that they have been prepared, packed, or held under conditions that do not meet cGMP requirements for dietary supplements.
NatRelief / Natural Relief Inc. (October 18, 2023)
FDA WARNING LETTER EXCERPT
Omar F. Ashraf, Owner
Natural Relief Inc.
901 State Route 541
Coshocton, OH 43812-9769
CMS # 659048
Dear Mr. Ashraf:
The U.S. Food and Drug Administration (FDA) inspected your facility located at 901 State Route 541 Coshocton, OH 43812-9769 from March 14, 2023- April 14, 2023. Based on our inspection, subsequent review of product labeling collected during the inspection, and review of your firm’s website, www.natrelief.com, we have identified serious violations of the Federal Food, Drug, and Cosmetic Act (the Act) and applicable regulations. You can find the Act and FDA regulations through links on the FDA’s home page at http://www.fda.gov.
Unapproved New Drugs and Misbranded Drugs
FDA reviewed your website, www.natrelief.com in June 2023 and determined that you take orders for Edema NatRelief 6 and BP NatRelief 11. The claims on your website, establish that your Edema NatRelief 6 and BP NatRelief 11 products are drugs under section 201(g)(1)(B) of the Act [21 U.S.C. 321(g)(1)(B)] because they are intended for use in the cure, mitigation, treatment, or prevention of disease. As explained further below, introducing or delivering these products for introduction into interstate commerce for such uses violates the Act.
Gadget Island Inc. (July 21, 2023)
Naeem Azizian
President/CEO
Gadget Island, Inc.
PO Box 981630
West Sacramento, CA 95798
PO Box 1156
Newark, CA 94560
RE: 658733
Dear Naeem Azizian:
This letter is to advise you that the United States Food and Drug Administration (FDA) reviewed your website at the Internet address, www.gearisle.com, between January and July 2023, where you took orders for “NUX Male Enhancement,” “DYNAMITE Male Sexual Enhancement,” and “ProPower Knight Plus 2550mg.”1 In addition, FDA has obtained samples and labeling of your “NUX Male Enhancement,” “DYNAMITE Male Sexual Enhancement,” and “ProPower Knight Plus 2550mg” products. As described below, these products are unapproved new drugs sold in violation of sections 505(a) and 301(d) of the Federal Food, Drug, and Cosmetic Act (FD&C Act), 21 U.S.C. 355(a) and 331(d). Furthermore, your “NUX Male Enhancement,” “DYNAMITE Male Sexual Enhancement,” and “ProPower Knight Plus 2550mg” products are misbranded drugs under section 502 of the FD&C Act, 21 U.S.C. 352, and sold in violation of section 301(a) of the FD&C Act, 21 U.S.C. 331(a).
FDA confirmed through laboratory analyses that samples of your “NUX Male Enhancement,” “DYNAMITE Male Sexual Enhancement,” and “ProPower Knight Plus 2550mg” products each contain the undeclared pharmaceutical ingredients tadalafil and sildenafil. Tadalafil and sildenafil are phosphodiesterase type-5 (PDE-5) inhibitors and the active ingredients in the FDA-approved prescription drugs Cialis and Viagra, respectively, used to treat erectile dysfunction (ED). These undeclared ingredients may interact with nitrates found in some prescription drugs, such as nitroglycerin, and may lower blood pressure to dangerous levels. Men with diabetes, high blood pressure, high cholesterol, or heart disease, often take nitrates.
Source:https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/gadget-island-inc-658733-07212023
Sobrenix, LLC, Kyle Armstrong and Kyle Dilger (July 19, 2023)
The Federal Trade Commission is taking action under the FTC Act and the Opioid Addiction Recovery Fraud Prevention Act of 2018 (OARFPA) against the makers of Sobrenix, which was marketed to reduce and even eliminate alcohol cravings and consumption.
According to the FTC’s complaint, the makers, a company called Rejuvica and its owners, Kyle Armstrong and Kyle Dilger, made numerous unsubstantiated and false claims about Sobrenix, a liquid tincture made with a blend of kudzu root and other herbs and vitamins, and used paid endorsers in deceptively formatted advertising. The defendants also used bogus review sites – including one touting Sobrenix – to deceive consumers about their products.
As a result of the FTC’s suit, the defendants have agreed to a proposed court order that would permanently ban them from making any unsubstantiated claims about health care products or services, as well as require them to pay $650,000 to the FTC to be used to provide refunds to consumers.
Source: https://www.ftc.gov/news-events/news/press-releases/2023/07/ftc-takes-action-against-makers-sobrenix-supplement-deceptively-claimed-reduce-alcohol-cravings
Hekma Center, LLC (June 1, 2023)
FDA WARNING LETTER EXCERPT
Mr. Younis
Hekma Center, LLC
24A Trolley Square #2131
Wilmington, DE 19806
RE: CMS # 637652
Dear Mr. Younis:
This letter is to advise you that the U.S. Food and Drug Administration (FDA) reviewed your website at https://www.hekmac.com in February 2023 and has determined that you take orders there for your Natural Supplements for Anemia, Lymf (Galium aparine), Natural Supplements for Cardiomyopathy, Magic1 (Moringa oleifera), Natural Supplements for Diabetes Mellitus, Cinna (Cinnamomum Zeylanicum), Natural Supplements for High Blood Pressure – Hypertension, kraph (Apium graveolens), Natural Supplements for Stroke – CVA, Cam1 (Silybum marianum), Danshen (Red sage), Multiple Sclerosis Package, and Smile Package products.
We also reviewed your social media websites, https://www.facebook.com/hekmac.supplement, https://www.instagram.com/hekma.c, https://twitter.com/hekma_center, and https://www.youtube.com/channel/UC_eIcBlLWbVkaj96gurUDdw, each of which directs consumers to your website, https://www.hekmac.com, to purchase your products. The claims on your website and social media websites establish that your products are drugs under section 201(g)(1)(B) of the Federal Food, Drug, and Cosmetic Act (the Act) [21 U.S.C. § 321(g)(1)(B)] because they are intended for use in the cure, mitigation, treatment, or prevention of diseases. As explained further below, introducing or delivering these products for introduction into interstate commerce for such uses violates the Act. You can find the Act and FDA regulations through links on FDA’s home page at www.fda.gov.
Examples of some of the website claims that provide evidence that your products are intended for use as drugs include the following:
Natural Supplements for Anemia
On your “Natural Supplements for Anemia” product page at https://www.hekmac.com/en.shop2/supplements-for-anemia:
“Supplement package for Anemia – Iron Deficiency”
“All products are safe for use and are very potent in helping people with Anemia.”
“Indicated for: People with Anemia”
“Many scientific studies have proven the medicinal properties and the efficiency of these herbs in helping with Anemia naturally without causing any side effects.”
“Patients with acute anemia should add royal sidr product (sidr honey with royal jelly) [an ingredient in your Multiple Sclerosis Package] to the package above.”
Source: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/hekma-center-llc-637652-06022023
Quality Supplement Manufacturing, Inc. (December 13, 2022)
FDA WARNING LETTER EXCERPT
300 N. Macarthur Blvd.
Oklahoma City, OK 73127
RE: 637182
Dear Raymundo Osuna,
The U.S. Food and Drug Administration (FDA) conducted an inspection of your facility located at 300 N. Macarthur Blvd, Oklahoma City, Oklahoma, from June 7, 2022, through June 13, 2022. Based on inspectional findings and a review of your product labels, we have identified serious violations of the Federal Food, Drug, and Cosmetic Act (the Act) and applicable regulations. You can find the Act and FDA regulations through links on the FDA’s home page at www.fda.gov.External Link Disclaimer
We received your written response, dated July 5, 2022, to the Form FDA 483, Inspectional Observations, issued to you at the close of the inspection. We have reviewed that document and our comments are listed following each of the significant violations.
Adulterated Dietary Supplements
The inspection of your facility revealed serious violations of the FDA’s regulations for Current Good Manufacturing Practice (CGMP) in Manufacturing, Packaging, Labeling, or Holding Operations for Dietary Supplements, under Title 21, Code of Federal Regulations (CFR), Part 111 (21 CFR Part 111). Based on the inspection, we determined that your products, including your (b)(4), and (b)(4) products are adulterated within the meaning of section 402(g)(1) of the Act [21 U.S.C. § 342(g)(1)] in that they have been prepared, packed, or held under conditions that do not meet CGMP requirements for dietary supplements.
Source: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/quality-supplement-manufacturing-inc-637182-12132022
Artri King Recall (April 20, 2022)
FDA is warning consumers not to purchase or use products marketed with variations of the names “Artri” or “Ortiga” due to potentially dangerous hidden active drug ingredients not listed on the product label. FDA urges consumers taking these products to immediately talk to their health care professional (e.g., doctor) to safely discontinue use of the product because suddenly stopping these drugs may be dangerous.
These products are promoted for treating arthritis, muscle pain, osteoporosis, bone cancer, and other conditions and are sold on various websites and in some retail stores.
FDA laboratory analyses revealed certain Artri and Ortiga products contain the undeclared drug ingredients:
- Dexamethasone (a corticosteroid) that can cause serious adverse events, including infections, increased blood glucose (sugar) levels, changes in blood pressure, damage to bones, psychiatric problems, and adrenal dysfunction;
- Diclofenac sodium (an anti-inflammatory drug) that can lead to adverse cardiovascular events, such as heart attack and stroke, or serious gastrointestinal damage, including bleeding, ulceration, and fatal tears of the stomach and intestines, or liver toxicity including liver failure that can cause the need for a liver transplant or death
- Methocarbamol (a muscle relaxant) that can cause sedation, dizziness, and low blood pressure.
These drug ingredients, which are not listed on the product label, can also interact with other drugs a consumer is taking.
FDA has received adverse event reports, including of liver toxicity and death, associated with the use of Artri King products, since the agency issued its first warning about an Artri Ajo King product on January 5, 2022.
Source: https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-consumers-not-purchase-or-use-artri-and-ortiga-products-which-may-contain-hidden-drug
Salud Natural Entrepreneur (March 3, 2022)
On March 3, 2022, the United States Government, on behalf of FDA, filed a complaint against Salud Natural Entrepreneur, Inc. (“Salud”), as well as its owner, production manager, and quality control manager. The complaint alleged that the defendants violated the Federal Food, Drug and Cosmetic Act by distributing adulterated and misbranded dietary supplements and unapproved new drugs. The complaint also alleged that Salud did not comply with good manufacturing practices.
The complaint followed inspections of Salud’s facility in May 2021, during which FDA found that the company was not complying with Good Manufacturing Practice regulations for dietary supplements. During a previous inspection, FDA found that Salud had used ingredients that had tested positive for Salmonella. Further, the complaint alleged that Salud’s products did not comply with labeling requirements and made unapproved drug claims (e.g, Aloe Vera Juice as an “antiviral” and Liver Detox Tea as treating alcoholism and gallbladder pain).
On March 8, 2022 the U.S. Department of Justice announced that a consent decree had been issued by the Northern District of Illinois. As a result, the defendants are permanently enjoined from manufacturing, holding, or distributing any products that they claim may treat or cure disease, until the products comply with federal law.
Source: https://www.justice.gov/opa/press-release/file/1480861/download
Blackstone Labs / Aaron Singerman (January 27, 2022)
A South Florida man who founded a sports and dietary supplements retailer was sentenced today to 54 months in prison for conspiring to sell illegal anabolic steroids and other products marketed as dietary supplements that were unlawful under federal law.
According to court documents, Aaron Singerman, 41, of Delray Beach, Florida, founded and operated Blackstone Labs LLC, a Boca Raton-based sports and dietary supplements retailer. Singerman is the former CEO of Blackstone. U.S. District Court Judge William P. Dimitrouleas of the Southern District of Florida sentenced Singerman to 54 months in prison and ordered him to forfeit $2.9 million.
In pleading guilty, Singerman admitted to leading a conspiracy to sell products through Blackstone labeled as dietary supplements that were illegal under federal law because they were not approved by the FDA and were controlled substances. According to court documents, Blackstone defrauded the FDA as part of the scheme.
Blackstone Labs (November 19, 2021)
According to court documents, Phillip “PJ” Braun, 40, of Boca Raton, Florida, and Aaron Singerman, 41, of Delray Beach, Florida, founded and operated Blackstone, a Boca Raton-based sports and dietary supplements retailer. Braun is Blackstone’s CEO and former President, and Singerman is the former CEO of Blackstone.
On Nov. 17, Braun and Singerman pleaded guilty to conspiracy to distribute controlled substances, in violation of 21 U.S.C. §§ 841(a)(1), 841(b)(1)(E), and 846, and to selling unapproved new drugs, in violation of 21 U.S.C. §§ 331(d), 355(a), and 333(a)(2). On Nov. 19, Blackstone Labs pleaded guilty to the same charges as Braun and Singerman, as well as to one count of conspiracy to defraud the U.S. Food and Drug Administration (FDA) and to commit mail and wire fraud, in violation of 18 U.S.C. § 371.
In pleading guilty, Braun and Singerman admitted to leading a conspiracy to sell products through Blackstone that were labeled as dietary supplements but were actually controlled substances or drugs that were not approved by the FDA. Blackstone made the same admissions, and also admitted to having defrauded the FDA and consumers by selling illegal substances falsely labeled as dietary supplements.
The defendants specifically admitted that, from 2012 through 2017, they conspired to sell products that were unapproved new drugs and/or illegal controlled substances under the Designer Anabolic Steroid Control Act. The defendants admitted that they falsely characterized their products as safe and legal dietary supplements. In addition, they falsely represented that the products were made in “FDA approved” registered facilities that followed all required regulations, when in fact they were not. The defendants also admitted to controlling a supplement manufacturer that fraudulently imported raw ingredients for their products from China. Braun and Singerman both admitted to selling many other products in violation of the Food, Drug and Cosmetic Act, including synthetic stimulants DMAA and DMBA, and the “nootropic” chemical picamilon. The defendants ignored injury complaints from consumers and did not notify the FDA of complaints, even when required by law. As part of their plea agreements, the defendants also agreed to forfeit all proceeds of these crimes, with Braun forfeiting $3 million, Singerman forfeiting $2.9 million and Blackstone forfeiting $1 million.
Editor’s Note: According to their website www.blackstonelabs.com accessed January 15, 2024, several products continue to be sold by this company which contain illegal supplement ingredients, such as Phenibut and steroid-like compounds.
Source:
https://www.justice.gov/opa/pr/blackstone-labs-founder-sentenced-conspiracy-sell-anabolic-steroids-and-unlawful-dietary
Quickwork LLC and Eric A. Nepute (April 15, 2021)
Nutritional supplement company Quickwork LLC and one of its managers, Eric Anthony Nepute, have agreed to injunctions and to pay civil penalties to resolve a lawsuit alleging they deceptively marketed vitamin supplements during the COVID-19 pandemic, in violation of the Federal Trade Commission Act and the COVID-19 Consumer Protection Act. The resolution of this lawsuit follows an order issued by the U.S. District Court for the Eastern District of Missouri on July 19, awarding partial summary judgment to the government.
In a complaint filed on April 15, 2021, the government alleged that the defendants made misleading and unsubstantiated advertising claims that their Vitamin D and Zinc supplements could be used to treat or prevent COVID-19, and in fact provide equal or better protection against COVID-19 than the available COVID-19 vaccines. The complaint also alleged that the defendants had mischaracterized the results of scientific studies to support some of their claims.
In an order entered on Nov. 14, 2022, Quickwork agreed to an injunction and a $1 million civil penalty, partially suspended due to an inability to pay. On July 19, the court granted partial summary judgment against Nepute, finding that there was no reasonable basis in the record to support claims that Zinc can treat or prevent COVID-19, or that Vitamin D or Zinc provide equal or better protection against COVID-19 than the available COVID-19 vaccines. In an order entered on Aug. 2, Nepute agreed to an injunction and to pay $80,000 in civil penalties.
The court’s injunctions prohibit the defendants from advertising that their supplements can prevent, cure, mitigate, or treat COVID-19 without competent and reliable scientific evidence to support such claims. The defendants are also banned from misrepresenting the results of COVID-19 research in their advertising. The defendants agreed to pay damages in the event that they make prohibited representations in the future.
Source: https://www.justice.gov/opa/pr/permanent-injunctions-and-judgment-over-1-million-civil-penalties-entered-case-deceptive
Hi Tech Pharmaceuticals – Product Recall (May 12, 2021)
Hi-Tech Pharmaceuticals. Inc. of Norcross, GA is recalling Lot # 001211197, Exp. 12/25 of Lipodrene w/25mg Ephedra Extract Dietary Supplement due to the presence of 1,4-dimethylamylamine (DMAA). The FDA has warned that DMAA is dangerous because it can narrow blood vessels and arteries and cause a corresponding rise in blood pressure or other cardiovascular problems, such as: Shortness of breath, Arrhythmias, Elevated blood pressure, Tightening in the chest, and Heart attack. ( https://www.fda.gov/food/dietary-supplement-products-ingredients/dmaa-products-marketed-dietary-supplements) Hi-Tech Pharmaceuticals, Inc. is conducting a continuing investigation on the problem.
There have been no reported illnesses to date.
The voluntary recall was the result of FDA analysis that showed the presence of 1,4-dimethylamylamine in one lot of Lipodrene. Customers who have purchased Lipodrene Lot # 001211197 are advised to stop using this lot of product immediately and return it to the place of purchase for a full refund. Lipodrene Lot # 001211197 was purchased by and distributed through wholesale and direct sales in the U.S. and Puerto Rico, and through online sales for both personal use and retail sales.
Retailers who have any of these products should remove them from the shelves and return them to Hi-Tech immediately. Wholesalers or distributors should alert their customers to the recall and have them return any product back to the place of purchase or to Hi-Tech Pharmaceuticals. Hi-Tech will immediately replace any returned items with product from a different lot.
———————-
“A manufacturer of dietary supplement products argued Tuesday that it’s unjust for a district court to enforce a $40 million judgment against the company in a long-running battle with the Federal Trade Commission.
In response, a panel of appellate court judges pushed back against arguments made by Robert Parsley, a lawyer representing Hi-Tech Pharmaceuticals and its owner Jared Wheat, who are both under criminal indictment in an unrelated case scheduled to go to trial in January.
In 2017, Senior U.S. District Court Judge Charles A. Pannell Jr. held Wheat, Hi-Tech and its sales executive Stephen Smith “jointly and severally liable” for $40 million in sanctions, following a determination that Hi-Tech violated a 2008 injunction that required substantiation—or “competent and reliable scientific evidence”—for its advertising claims.”
———————
Georgia-based Hi-Tech and FTC, the more than a century-old agency responsible for policing deceptive advertising practices, have been at odds for two decades.
FTC in 2004 sued Hi-Tech, Wheat and others for false advertising of dietary supplement products, in violation of a federal statute (the “FTC Act”) that prohibits deceptive business practices and false advertisements.
In June 2008, in a 99-page order entered in the U.S. District Court for the Northern District of Georgia, Pannell found the defendants violated the law, and he held them “jointly and severally liable” for $15.9 million.
Later that same year, Pannell granted a permanent injunction against the corporate and individual defendants, prohibiting claims of rapid or substantial weight loss, for example, without competent and reliable scientific evidence.
Although the judgment was affirmed on appeal, the fight between Hi-Tech and the government did not end by any stretch of the imagination.
“Hi-Tech utterly failed to comply with any aspect of the district court’s 2008 injunction,” neither paying “the monetary judgment” nor stopping “its false advertising,” according to the government’s brief filed in May 2022 with the Eleventh Circuit. Instead of satisfying the judgment, which it had the resources to do, Hi-Tech “went on a spending spree, acquiring other dietary supplement companies, buying luxury goods, and otherwise dissipating millions of dollars,” the court filing alleged.
Source: https://www.naturalproductsinsider.com/supplement-regulations/hi-tech-pharmaceuticals-ftc-clash-yet-again-over-40-million-in-sanctions
The former principal of a multilevel marketing organization selling a mushroom-infused coffee has been permanently banned from operating such a business and has been slapped with a $7.3 million fine.
A federal court brought the contempt of court ruling against James D. ‘Jay’ Noland Jr. on the basis of a complaint by the Federal Trade Commission (FTC).
Noland was found to have illegally owned and operated two pyramid schemes—Success by Health (SBH) and VOZ Travel. He was also judged to have violated a previous court order barring him from operating a pyramid scheme and from misrepresenting the income potential that participants in the scheme could aspire to.
MCACO Ltd. (April 21, 2022)
FDA WARNING LETTER EXCERPT
1610 W. Polo Rd
Grand Prairie, TX 75052
Dear Mr. Eric Aguayo,
This is to advise you that the U.S. Food and Drug Administration (FDA) conducted an inspection of your facility, located at 1610 W. Polo Rd, Grand Prairie, TX from November 4, 2021, through December 7, 2021. Based on the inspectional findings we have identified serious violations of the Federal Food, Drug, and Cosmetic Act (the Act) and applicable regulations. You can find the Act and FDA regulations through links on the FDA’s home page at http://www.fda.gov.External Link Disclaimer
Adulterated Dietary Supplements
The inspection of your facility revealed serious violations of FDA’s regulations for Current Good Manufacturing Practice (CGMP) in Manufacturing, Packaging, Labeling, or Holding Operations for Dietary Supplements under Title 21, Code of Federal Regulations, Part 111 (21 CFR 111). These violations cause your dietary supplement products to be adulterated within the meaning of section 402(g)(1) of the Act [21 U.S.C. § 342(g)(1)] in that they have been prepared, packed, or held under conditions that do not meet CGMP requirements for dietary supplements. FDA acknowledges the receipt of your e-mail correspondence dated December 30, 2021, written in response to the Form FDA 483, Inspectional Observations, issued to you at the close of the inspection. We address your response below, in relation to each of the noted violations.
Source: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/mcaco-ltd-624606-04212022
S.K. Laboratories / Sitesh Patel (February 19, 2021)
A federal court in Texas sentenced a former dietary supplement company executive to prison for his role in fraudulently selling popular workout supplements, the Justice Department announced today.
On Feb. 19, 2021, U.S. District Judge Sam A. Lindsay sentenced former S.K. Laboratories Vice President Sitesh Patel, 37, of Irvine, California, to 41 months’ imprisonment and one year of supervised release. The court previously ordered Patel’s former company, S.K. Laboratories, to forfeit $6 million in connection with the case.
According to documents filed in the case, Patel played a key role in developing and manufacturing the popular workout and weight loss supplements known as Jack3d and OxyElite Pro, which were distributed by Dallas-based USPlabs. In pleading guilty in 2019 to conspiracy to introduce misbranded food into interstate commerce, Patel and several of his co-defendants admitted that they imported substances with false and misleading labeling to avoid law enforcement and regulatory agency attention. Patel also pleaded guilty to introduction of misbranded food into interstate commerce. The misbranding charges relate in part to OxyElite Pro, which was recalled in 2013 in the wake of an investigation by the U.S. Food and Drug Administration (FDA) into whether the supplement caused liver injuries in consumers. An indictment returned by a Dallas federal grand jury in 2015 against Patel and four other individuals associated with USPlabs alleged that the defendants sold some of their products without determining whether they would be safe to use…
The court previously sentenced Jacobo Geissler, 44, of University Park, Texas, the CEO of USPlabs, to 60 months’ imprisonment, and Jonathan Doyle, 41, of Dallas, the president of USPlabs, to 24 months’ imprisonment for their roles in the fraud. The court also sentenced defendants Cyril Willson, 40, of Ralston, Nebraska, a former consultant for USPlabs, and Matthew Hebert, 42, of Dallas, a co-owner of the company, to 18 months’ and 15 months’ imprisonment, respectively. In addition, USPlabs was ordered to pay $4.7 million in criminal forfeiture.
Source: https://www.justice.gov/opa/pr/dietary-supplement-executive-sentenced-scheme-fraudulently-sell-popular-dietary-supplements
ABH Pharma, Inc. / ABH Nature’s Products, Inc. / StockNutra.com Inc. and Mohammed Jahirul Islam (December 26, 2019)
The U.S. District Court for the Eastern District of New York permanently enjoined ABH Nature’s Products, Inc., ABH Pharma, Inc., StockNutra.com, Inc. (together, “ABH”), each of Edgewood, New York, and their owner, Mohammed Jahirul Islam (“Islam”) of Flushing, New York from distributing adulterated and misbranded dietary supplements in violation of the Federal Food, Drug, and Cosmetic Act, the Department of Justice announced today.
The injunction requires ABH and Islam to destroy, within 15 days, dietary supplements that are in their possession, custody, or control. The injunction also orders ABH and Islam to implement several consumer safety measures before resuming the manufacturing or distributing of dietary supplements. Those measures include hiring an independent expert to inspect ABH’s facility and certify that the facility has corrected all deficiencies and implemented current good manufacturing practices. It also mandates that the defendants engage a labeling expert to review their product labeling and certify that claims on their products comply with the law.
The injunction stems from a complaint the Department filed on Nov. 21, 2019, at the request of the U.S. Food and Drug Administration (FDA). According to the complaint, ABH and Islam manufactured, prepared, labeled, packed, held, and distributed dietary supplements under conditions that failed to comply with current good manufacturing practice regulations. In particular, the complaint alleged that the FDA had observed several critical deviations from current good manufacturing practice regulations during its inspections of ABH’s manufacturing facility, including failures to verify that certain dietary supplements met the product’s specifications for identity, purity, strength, and composition; to implement a production system that ensured the quality of the supplements; to include necessary information in its production records; and to properly review and investigate a consumer complaint.
In addition, the complaint alleged that ABH and Islam further violated the Federal Food, Drug, and Cosmetic Act by distributing unapproved and misbranded “new drugs” into interstate commerce. For instance, as alleged in the complaint, ABH made claims on product labeling that such products could be used to treat such medical conditions as cancer, heart disease, HIV and AIDS, even though the FDA had not approved those products for such purported uses, nor were there any published adequate and well-controlled investigations showing that such products are generally recognized as safe and effective for any use.
The defendants agreed to resolve the complaint and be bound by a consent decree of permanent injunction. The court adopted the agreement and entered the injunction.
Source: https://www.justice.gov/opa/pr/federal-district-court-orders-new-york-company-stop-distributing-adulterated-and-misbranded#
Banned Supplements on Amazon (2023)
The list of supplements prhobited by Amazon.com is located at this page.
Visit the source page at the Amazon website for an updated list.


Sorry, the comment form is closed at this time.