“Verification” is the 2016 buzzword for food and supplements, due to the sequence of food safety crises that arguably started with salmonella in peanut butter in the early 2000s.
Recently, FSMA and the “Identity Crisis” for botanical ingredients in supplements have renewed the requirement for verification of quality and safety practices in the supply chain: raw materials, manufacturing practices and test methods being three big areas of focus.
“Trust But Verify” is attributed to President Reagan and later FDA and quality assurance folks. Although it is a well meaning mantra, doesn’t it make verification seem optional? Shouldn’t we verify BEFORE trusting?
We do know that trust disappears soon after a failure to verify becomes apparent.
From Salmonella in peanut butter, to misidentified plant extracts, to illegal levels of contaminants in your food or supplement, verification is how trust is ensured.
While trust is the ultimate goal, verification comes first.
#verifythentrust
First published on LinkedIn, April 2016
 Why should your customer pick your product or ingredient over all the others? Because they are able to communicate it’s value.
Why should your customer pick your product or ingredient over all the others? Because they are able to communicate it’s value.
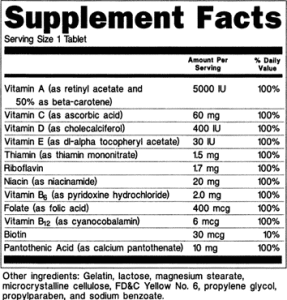 Validation of matrix-specific methods across multiple laboratories address these challenges, however few methods have been validated to the extent required to be confident in the results. An example from the nutrition field: the inherent challenges in quantification of vitamin D (a pure compound and age-old vitamin, no less!)
Validation of matrix-specific methods across multiple laboratories address these challenges, however few methods have been validated to the extent required to be confident in the results. An example from the nutrition field: the inherent challenges in quantification of vitamin D (a pure compound and age-old vitamin, no less!) The true test of scientific validity is when multiple labs running different methods achieve the same result, especially when they are blinded as to the expected result.
The true test of scientific validity is when multiple labs running different methods achieve the same result, especially when they are blinded as to the expected result.

 Today’s analytical technology to measure analytes in complex mixtures is way ahead of the not-too-distant past, but now we understand a mitigating factor: that with greater power and resolution comes an increasing number of factors that may cause test results to be inaccurate or imprecise.
Today’s analytical technology to measure analytes in complex mixtures is way ahead of the not-too-distant past, but now we understand a mitigating factor: that with greater power and resolution comes an increasing number of factors that may cause test results to be inaccurate or imprecise.
 Exciting stuff, all this mystery, which we eventually find answers to through validation and repetitious testing. While it’s difficult to predict analytical uncertainty, the point is to control it to the extent possible, hopefully to within 5-10% of your expected result — not bad compared to the 20% tolerance limit required by pharmaceuticals.
Exciting stuff, all this mystery, which we eventually find answers to through validation and repetitious testing. While it’s difficult to predict analytical uncertainty, the point is to control it to the extent possible, hopefully to within 5-10% of your expected result — not bad compared to the 20% tolerance limit required by pharmaceuticals.